New paper – ‘The SARS-CoV-2 protein ORF3c is a mitochondrial modulator of innate immunity’, led by Hazel Stewart from Andrew Firths lab at Cambridge and Yongxu Lu is now published in iScience. You can read the paper here.
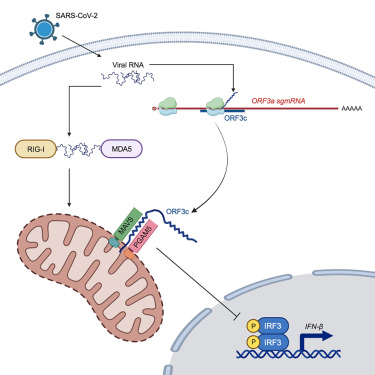
The SARS-CoV-2 genome encodes a multitude of accessory proteins. Using comparative genomic approaches, an additional accessory protein, ORF3c, has been predicted to be encoded within the ORF3a sgmRNA. Expression of ORF3c during infection has been confirmed independently by ribosome profiling. Despite ORF3c also being present in the 2002–2003 SARS-CoV, its function has remained unexplored. Here we show that ORF3c localises to mitochondria, where it inhibits innate immunity by restricting IFN-β production, but not NF-κB activation or JAK-STAT signalling downstream of type I IFN stimulation. We find that ORF3c is inhibitory after stimulation with cytoplasmic RNA helicases RIG-I or MDA5 or adaptor protein MAVS, but not after TRIF, TBK1 or phospho-IRF3 stimulation. ORF3c co-immunoprecipitates with the antiviral proteins MAVS and PGAM5 and induces MAVS cleavage by caspase-3. Together, these data provide insight into an uncharacterised mechanism of innate immune evasion by this important human pathogen.
This work was first released on bioRxiv, and you can read the preprint here.

Comments are closed, but trackbacks and pingbacks are open.